Green Chemistry
“Green chemistry is the selection of less hazardous, more sustainable chemicals, and/or the design of chemical products or protocols involving chemicals that aim to reduce or eliminate the use or generation of hazardous substances.” - My Green Lab
Green Chemistry is not a sub-discipline of chemistry, but rather a set of principles that encourage the use and/or creation of less hazardous and more sustainable chemicals and chemical products. There are 12 principles that guide designing green chemical reactions. The principles of Green Chemistry are focused on reducing hazards (to human health and the environment), materials, waste, and cost. Green Chemistry typically goes hand-in-hand with laboratory safety. When you use safer chemicals in the lab, you decrease the risk of potential hazards.
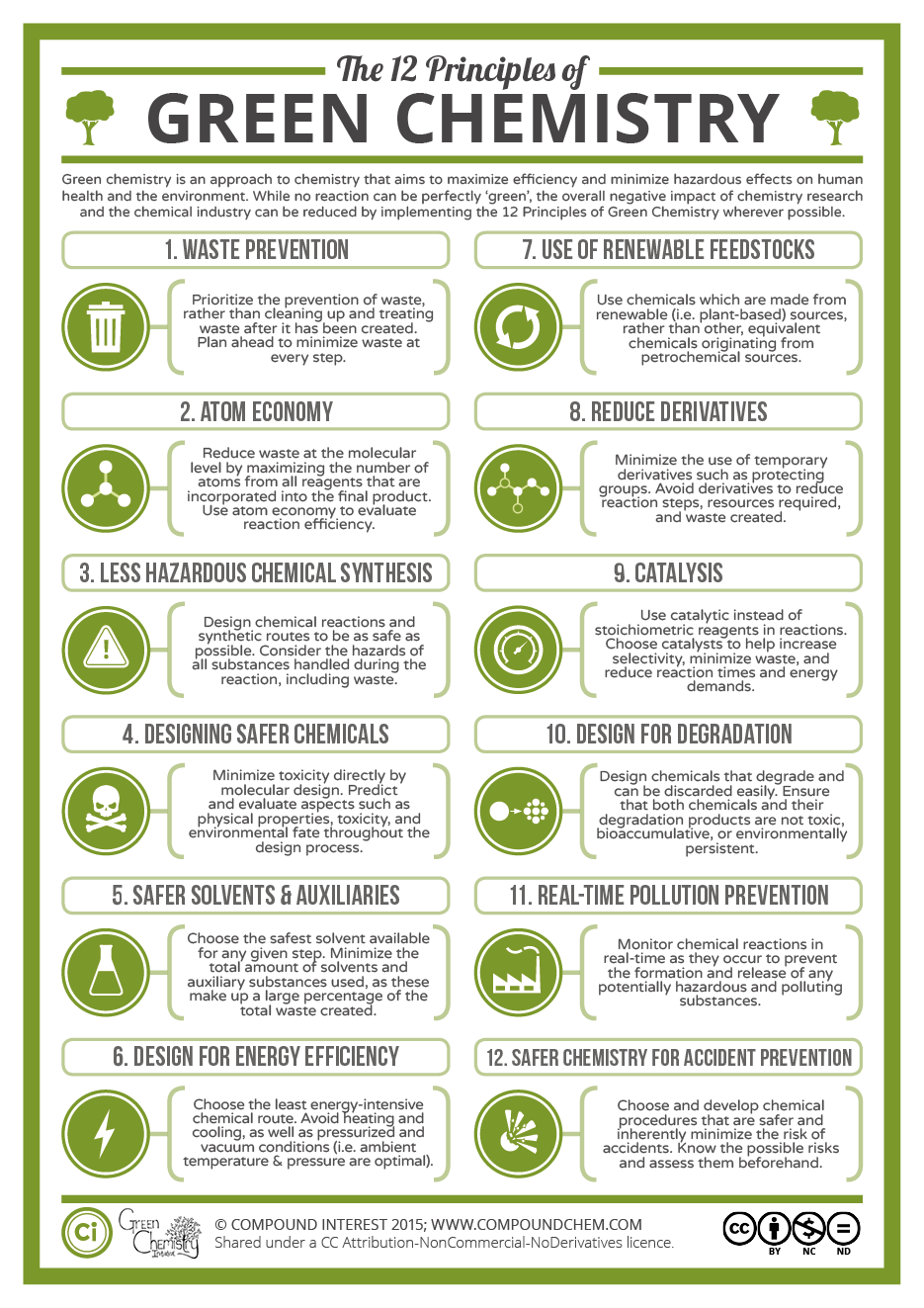
Image from: Compound Interest
Getting Started
Practicing Green Chemistry starts with identifying areas and procedures in which chemical hazards can be reduced and sustainability increased. This is lab-specific, but below are a few good questions to ask yourself to get started.
1. Are there any processes that involve the use or creation of hazardous materials?
- Be sure to consider reagents, solvents and by-products.
- For specific information on hazards and handling of chemicals, refer to the chemical’s SDS or the EPA website.
- You can also do a quick search on ECHA’s REACH candidate list or ChemSec SIN (Substitute It Now) List. The ChemSec SIN list also provides marketplace alternatives.
- Do a literature search to see if there any alternative procedures that avoid use of these materials.
- If you understand the reaction chemistry of your process, refer to the resources in the section below to search for greener materials.
2. Does your lab use any solvents in large quantities?
- See the section below for resources on solvent recycling.
3. Are there procedures that use large quantities of materials or generate a great deal of waste?
- To evaluate, you can calculate metrics such as reaction mass efficiency (RME), atom economy (AE), mass intensity (ME) and E-factor. These can apply to an entire process, or a single step.
- When comparing procedures, keep in mind the nature of the materials in terms of hazards, renewability, and cost.
Resources
1. ‘Greener’ Choice of Reagents
- The ACS Reagent Guide allows scientists to:
- Research by the type of reaction (e.g. oxidations to aldehydes and ketones)
- Compare different reagents using a Venn Diagram
- Click on individual reagents and get information about the mechanism, literature, and a “Green Review”, including:
- Atom efficiency
- Safety Concerns
- Toxicity and environmental/aquatic impact
- Cost, availability & sustainable feedstocks
- Sustainable implications
**NOTE: This tool requires users register for an account with the website.
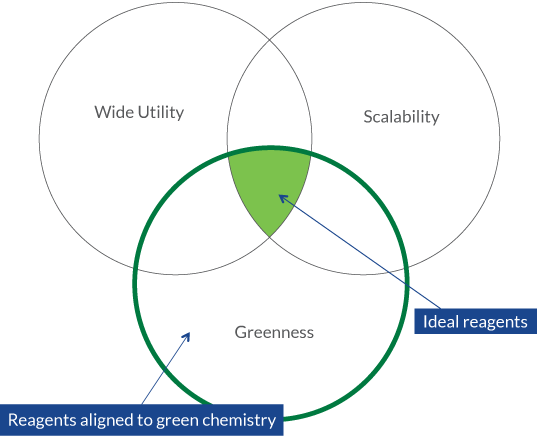
Image from: ACS
2. ‘Greener’ Choice of Solvents
ACS offers a Solvent Selection Tool. This tool is most useful for those who have a good understanding of the properties they need in a solvent and are looking for alternatives that better facilitate their reactions, are safer, and have fewer environmental impacts. The tool displays the information using principle component analysis (PCA): the farther apart solvents are on the map, the more physically and chemically different they are anticipated to be. View the Hints and Tips page for more information on how to use the tool.
3. Choice of Purification Technique
Be thoughtful about what purification techniques are necessary to achieve your experimental goal. Some separation methods can be replaced by ‘greener’ methods. For example, CO2 recycling supercritical fluid chromatography (SFC) can be used in some instances in place of high-performance liquid chromatography (HPLC) (ACS).
4. Solvent Recycling
Millipore Sigma has a ReCyclerTM program for bulk quantities of solvents such as acetone, chloroform, isopropyl alcohol, and methanol. Solvent is supplied in returnable, reusable containers. Labs can set up a regular delivery, or just return the container when empty and have it refilled.
Additional Resources
- ACS Green Chemistry Guide for Medicinal Chemistry - covers reagents, solvents, purification techniques and more.
- MilliporeSigma DOZN™ - an interactive web app which provides a score as to how well a product aligns with each of the 12 principles of green chemistry
- Chem21 - This is an online platform to learn the fundamentals of green chemistry geared toward the pharmaceutical industry.
- Quartzy - You can use this free online resource to manage your lab’s inventory and track order requests. This can help minimize over-purchasing and, consequently, chemical waste.
- Beyond Benign - Provides great resources for Green Chemistry education.
- Undergraduate Organic Chemistry Lab Green Chemistry Guide - This is a comprehensive teaching guide for undergrad labs featuring green chemistry alternative experiments, developed by Beyond Benign, MyGreenLab and Millipore Sigma.
If you have found a green alternative for a chemical process, tell us about it! We want to recognize your efforts and make this information available to the Green Labs community. There are likely other labs who perform the same procedure, and we want to encourage them to go green like you!
